From internal pipelines, drains are transported by external ...
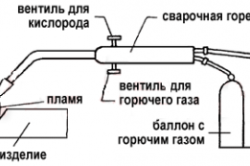
Gas welding is widely in demand in production and in the household. More and more private traders who prefer to independently perform various works do this using sophisticated techniques. This allows them to perform complex tasks and carry out a variety of projects.
For this reason, do-it-yourself gas welding is of interest to home craftsmen. But before picking up the burner, you need to find out how and how to do it.
Gas welding is the process of melting the base and filler metals on the edges of the parts as a result of exposure to a burner flame. The choice of chemical composition of filler bars depends on the physicochemical characteristics of the base metal.

Figure 1. Technology gas welding.
The flame is maintained by supplying gas to the burner together with technically pure oxygen (Fig. 1). Adding the latter makes the fire suitable for use in welding. Moreover, by what proportion oxygen occupies, the property of fire and its practical application are determined.
According to the ratio of gases, the gas welding flame is divided into three types:
The flame of the first type (it is also called normal) contains equal proportions of acetylene and oxygen. Oxidative fire is formed with an excess of oxygen, and carburizing is characterized by an excess of acetylene.
Unlike electric arc welding, gas welding provides smooth heating of metal edges.
With its help, with different methods of soldering and surfacing, steel parts are processed having a thickness of 0.2-5 mm, various types of tool steels, as well as non-ferrous metals and cast iron. All of these metals must be welded by soft and slow heating.
The flame of a gas burner is created due to the combustion of working gases under the influence of oxygen. The purity of the latter should be at least 98%.
In gas welding, several gaseous chemical elements are used as combustible gases. These are acetylene, methane, hydrogen, propane and propane-butane mixtures, pairs of lighting kerosene and gasoline. All of these substances burn well in the open air.
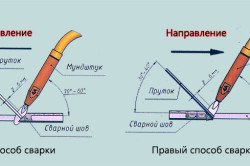
Figure 2. Welding methods - right and left.
A feature of all the gases mentioned is that they themselves do not generate the very high temperature necessary for the rapid melting of metal structures. To do this, they need additional oxygen flow.
The most popular among these gaseous substances today is acetylene gas. It is actively formed as a result of a chemical reaction when calcium carbide is combined with ordinary water. Interacting with an oxygen stream, acetylene at the time of combustion "gives out" a temperature of up to 3200-3400 ° C. To obtain it, special generators are used, which are currently widely produced by industry.
In a gas welding apparatus, the connection of acetylene with oxygen occurs in a special mixing part of the burner. Both gases are supplied to this chamber through hoses separately: acetylene from the generator, and oxygen from the cylinder, which traditionally has either blue or blue color. The oxidizer is contained in a container under a pressure of 3-4 atmospheres.
It should be noted that the constituent components gas mixture served under different pressures (oxygen has more). Therefore, when oxygen enters the central feed channel of the burner, its advancement creates a strong vacuum, due to which acetylene, pumped under lower pressure, is gravity-sucked into the channel. Here, in the mixing department, the gases are mixed, react and through the tip go out to the welding point.
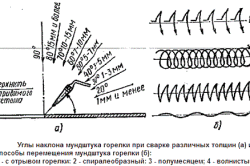
Figure 3. Tilt angles of the nozzle of the torch when welding various thicknesses.
In order to properly perform welding operations, it is necessary to understand the principles of welding operations and the sequence of actions of a gas welder. The technology of these works includes preparatory operations, including processing the welded edges of metal billets and choosing a welding method, setting the gas torch in the proper position, as well as determining all the required parameters of the gas welding apparatus, including the power of the fire jet and the diameter of the wire additive.
In preparation for welding, the metal edges of the workpiece should be cleaned of various contaminants, scale and oil. On a special machine or, if the machine is not available, using an ordinary chisel (you can also use a pneumatic version of this tool), the bevel is made at the edges, which is necessary to fill the future weld with a molten welding additive.
During operation, it is very important that the position of the elements to be welded is rigidly fixed. In order to ensure the impossibility of their movement relative to each other, prior to the main welding, grab the edges of the workpieces.
If we are talking about thin metal sheets and short seams, then tacks are made in lengths of 6-7 mm each, between them there should be unattached gaps with a length of about 70-100 mm. If thick metal parts are connected, and the seams are planned to be made long, the length of each tack should reach 25-30 mm at intervals between them of 300-500 mm.
Turning to welding, we note that its quality largely depends on the correct position of the torch with respect to the butt joint and on the direction of wiring along the joint. Here, the right and left variants of the direction of production of welding operations are distinguished (Fig. 2).
When using the movement of the working body of the gas-welding unit to the right, the wiring is carried out from left to right. In this case, the torch moves in front of the wire filler, and its flame is directed to the formed welding seam.
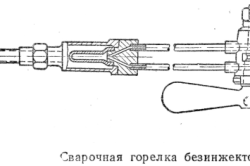
The left way, on the contrary, involves moving the burner from right to left. With this movement, the burner is located above the additive. As a result, the fire stream is directly directed at metal edges not welded to each other. Intensive heating of the edges takes place, which are thus prepared for subsequent high-quality welding.
It is worth noting that using the right method, metal parts with a thickness of more than 5 mm are connected, and ceiling welds are made. At the same time, vertical seams are formed in the left way, if welding is performed from bottom to top.
During gas welding, the torch tip and the filler rod should move relative to each other (Fig. 3) / The mouthpiece is moved along the seam and at the same time the suture axis, and the filler rod is gradually moved towards the mouthpiece.
Welding equipment must be in good condition. Otherwise, work is prohibited.
Transportation gas cylinders produced either by special stretchers, or on a specially designed trolley.
When working indoors, it is imperative to provide for breaks with access to fresh air.
When working in tanks, the presence of a second worker outside is mandatory.
The welder must have safety glasses.
Observing all these rules, you can do gas welding at a high level with your own hands.
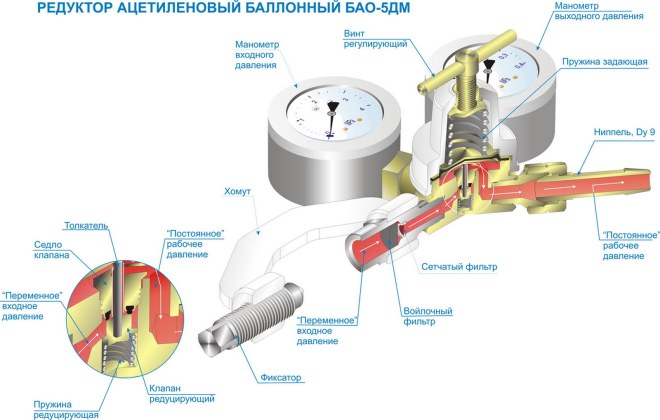
Oxygen at atmospheric pressure and ordinary temperature is a gas without color and odor, somewhat heavier than air. At atmospheric pressure and a temperature of 20 ° C, the mass of 1 m3 of oxygen is 1.33 kg. The combustion of flammable gases or vapors of flammable liquids in pure oxygen occurs very intensively, and a high temperature develops in the combustion zone. To obtain a welding flame with a high temperature necessary for the metal to melt at the welding site, combustible gas or a pair of combustible liquid is burned in a mixture with technically pure oxygen. If the combustion of gases occurs in air in which oxygen contains only 1/5 by volume (the remaining 4/5 are nitrogen and other atmospheric gases), the temperature of the welding flame will be much lower and the combustion process will be much slower than in technically pure oxygen. Oxygen itself is non-toxic, non-combustible and non-explosive, however, being the strongest oxidizing agent, it sharply increases the ability of other materials to burn, and at a very high burning speed - to explode. Industrial oxygen is extracted from atmospheric air, which is subjected to treatment in air separation plants, where it is cleaned of dust, carbon dioxide and dried from moisture. The air processed in the installation is compressed by the compressor to high pressure and cooled in heat exchangers until it is liquefied. Liquid air is separated into oxygen and nitrogen. The separation process occurs due to the fact that the boiling point of liquid nitrogen is lower than the temperature of liquid oxygen by 13 ° C. Nitrogen turns out to be a more boiling gas and evaporates first, so it is diverted from the air separation unit to the atmosphere. Liquid pure oxygen accumulates in the air separation apparatus. When oxygen is evaporated, they are filled with cylinders under pressure created by a compressor. Technical oxygen is transported in steel cylinders according to the requirements of existing regulatory documents or in auto-recipients at a pressure of 15 ± 0.5 MPa (150 ± 5 kgf / cm2) or 20 ± 1.0 MPa (200 ± 10 kgf / cm2) at 20 ° C. When filling the cylinders, their storage and transportation in the temperature range from -50 to +30 ° C, the gas pressure in the cylinder should correspond to that given in table. 49.
Table 49
Oxygen pressure in the cylinder depending on the ambient temperature
|
Gas temperature. SS |
Gas pressure in a cylinder, MPa (kgsUsm2) |
Tolerance. MPa (kgf cm :) |
Pressure gav in the tank. MPa (kgf cm3) |
Tolerance. MPa (kgf "cm3) |
|
15 MPa (150 kgf / cm5) arn 20 ° С |
20 MPa (200 kgf cm3) prn 20 "C |
|||
For welding and cutting, technical grade 1 oxygen is produced with a purity of at least 99.7% and grade 2 with a purity of at least 99.5%. When storing or transporting filled cylinders, the pressure in them must correspond to the ambient temperature. Storage and transportation of filled cylinders at temperatures above 60 ° C is not allowed. Oxygen cylinders must return to
filling with a residual pressure of at least 0.05 MPa (0.5 kgf / cm2).
Acetylene (C2H2) is a chemical compound of carbon with
hydrogen. It is a colorless flammable gas with a sharp characteristic
smell. Prolonged inhalation of acetylene causes dizziness, nausea, and sometimes severe general poisoning. Acetylene is lighter than air:
1 m3 of acetylene at 20 ° C and atmospheric pressure has a mass of 1.09 kg. Acetylene is an explosive gas. Temperature
self-ignition of acetylene is in the range of 240-630 ° C and depends on pressure and the presence of various impurities in acetylene. At
atmospheric pressure, a mixture of acetylene with air explodes when the content of acetylene in it is 2.2% or more, and in a mixture with oxygen at a content of 2.8% or more. Air acetylene explosion or
acetylene-oxygen mixture can occur from a spark, flame or strong local heating, therefore, handling calcium carbide and acetylene requires caution and strict adherence to safe work rules. In industry, acetylene is produced at
decomposition of liquid fuels, such as oil, kerosene, by electric arc discharge. A method of producing acetylene from natural gas (methane) is also used. A mixture of methane and oxygen is burned in special reactors at a temperature of 1300-1500 ° C. Concentrated acetylene is extracted from the resulting mixture with a solvent. Obtaining acetylene by industrial methods is 30-40% cheaper than from calcium carbide.
Industrial acetylene is pumped into cylinders, where it is located in the pores of a special mass dissolved in acetone. In this form, consumers receive balloon industrial acetylene. The properties of acetylene do not depend on the method of its preparation. The residual pressure in the acetylene cylinder at a temperature of 20 ° C should be 0.05-0.1 MPa (0.5-1.0 kgf / cm2). The working pressure in the filled cylinder should not exceed 1.9 MPa (19 kgf / cm2) at 20 ° C. For the safety of the filling mass, acetylene cannot be taken from the cylinder at a speed of 1700 dm3 / h. Let us consider in more detail the method of producing acetylene in a generator of calcium carbide. Calcium carbide is obtained by fusing coke and quicklime in electric arc furnaces at a temperature of 1900-2300 ° C, at which the reaction proceeds: CaO + 3C \u003d CaC2 + CO. The molten calcium carbide is poured from the furnace into the molds, where it cools. Then it is crushed and sorted into pieces from 2 to 80 mm in size. Finished calcium carbide is packaged in hermetically sealed drums or tin cans of 40; 100; 130 kg Calcium carbide should not contain more than 3% of particles less than 2 mm in size (dust). According to the relevant standard, the sizes (granulation) of pieces of calcium carbide are set: 2x8; 8x15; 15x25; 25x80 mm. When interacting with water, calcium carbide releases gaseous acetylene and forms slaked lime, which is a waste, in the residue. The decomposition of calcium carbide with water occurs according to the scheme:
From 1 kg of chemically pure calcium carbide, theoretically, 372 dm3 (liters) of acetylene can be obtained. Almost due to the presence of impurities in calcium carbide, the yield of acetylene is up to 280 dm3 (liters). On average, 4.3–4.5 kg of calcium carbide is consumed to produce 1000 dm3 (liters) of acetylene. Carbide dust decomposes almost instantly when wetted with water. Carbide dust can not be used in conventional acetylene generators, designed to work on lumpy calcium carbide. For the decomposition of carbide dust, generators of a special design are used. To cool acetylene during decomposition of calcium carbide, 5 to 20 dm3 (liters) of water are taken per 1 kg of calcium carbide. A “dry” method of decomposing calcium carbide is also used. For 1 kg of finely divided calcium carbide, 0.2-1 dm3 (liter) of water is supplied to the generator. In this process of slaking lime, it is obtained, not in the form of liquid sludge, but in the form of a dry “fluff”, the removal, transportation and disposal of which are greatly simplified. When welding and cutting metals, other combustible gases and vapors of flammable liquids can also be used. To heat and melt the metal during welding, it is necessary that the flame temperature is approximately 2 times higher than the temperature of the metal being welded. Therefore, it is advisable to use gases - substitutes for acetylene only when welding metals with a lower melting point than steel, such as aluminum, its alloys, brass, and lead. When cutting scrap metal use propane. Propane is a combustible gas that is produced during production. natural gas or in oil refining. Usually they get not pure propane, but with an admixture of butane up to 5-30%. This mixture is called propane-butane. For work, the propane-butane mixture is delivered to the consumer in a liquefied state in special cylinders. The transition of the mixture from a liquid to a gaseous state occurs spontaneously in the upper part of the cylinder due to the lower specific gravity of the gas in comparison with the liquefied mixture. Technical propane is heavier than air and has an unpleasant specific odor. Natural gas consists mainly of methane (98% purity), the rest is impurities in small amounts of butane and propane. The gas has a faint odor, so special smelling substances are added to detect a leak. Most often, methane is used in metal cutting. For the formation of a gas flame, other gases (hydrogen, coke oven and petroleum gases), combustible liquids (gasoline, kerosene, acetone, etc.) can also be used as fuel. Liquid fuels are less scarce, but require special containers for storage. For welding, cutting and soldering, the flammable liquid is converted into fumes by the flame of the tip of the torch or torch. The characteristics of various combustible gases and liquids used in various engineering industries and in the jewelry industry are given in table. fifty.
Table 50
Characteristic of flammable gases and liquids expressed in terms of acetylene coefficient
|
Name fuel |
Flame temperature ;! during combustion with oxygen, ° С |
Mass I and ’of fuel at 20 ° С and dailen; w 7 £ 0 mm Hg_st .. kg |
Coefficient of replacement |
Amount of oxygen supplied to the burner per 1 bg1 of fuel, m1 |
|
Gases: acetylene |
||||
|
pyrolysis |
||||
|
nefіyanoy |
||||
|
technical |
||||
|
natural |
||||
|
coke |
||||
|
shale |
||||
|
Kerosene Pairs |
March 22, 2017
The possibility of semi-automatic welding of materials in carbon dioxide was discussed in the middle of the twentieth century. Developed this technique N. Novozhilov and Lyubavsky K.V. - Soviet researchers. Due to the high degree of productivity, this welding method has become quite popular in the construction, manufacturing industry, and, of course, in everyday life, due to the low cost of carbon dioxide.
According to this technique, carbon dioxide, which provides protection in the connected area, is divided into O 2, carbon monoxide under the influence of the high temperature of the arc. As a result, the flow of the resulting gas mixture protects the zone of welding of the material from the negative effects of ambient air, interacts with carbon, iron.
To prevent the oxidation of CO 2, manganese is introduced into the rod for gas welding; silicon, which are chemically more active than iron, is the first to be oxidized. Therefore, while Mn, Si will be present at the junction of metal products, carbon, iron will not be oxidized.
To obtain high-quality welds when welding carbon steels, the proportion of manganese / silicon is taken 1/2. The resulting oxides of manganese and silicon do not dissolve in the weld pool during work, they form a fusible compound after reaction between themselves. This compound is easily removed from a metal in a liquid state.
Semi-automatic welding in carbon dioxide is carried out by direct current having reverse polarity, since the current of direct polarity negatively affects the stability of the arc (the weld will have defects).
The current of direct polarity is used in the case of deposition, but not welding, since it has a deposition coefficient of 1.7 times higher than this coefficient for a current having reverse polarity.
Welding can also be done on alternating current, but then an oscillator must be used in the circuit.
There are several types of welding. They differ among themselves by the high-temperature education technology, the purpose of which is to join, cut metals, their alloys. This can be accomplished with a gas flame, ultrasound or an electric arc. The principle of joining metals is based on the melting of the edges of individual metal structures for their further joining together, which results in a weld.
Depending on the gas used for welding, the temperature will vary. For example, when interacting with calcium carbide H 2 O, acetylene is released. During the reaction of this element with oxygen, the flame temperature can reach more than 3000ºС.
Welding gases are all butane, propane, benzene, MAF, kerosene, etc. When using any gas for welding, the presence of oxygen is mandatory - it is a combustion catalyst. O 2 must be clean and high quality. The maximum temperature indicator will depend on this.
In the gas composition, the presence of pure oxygen is mandatory, which makes it possible to obtain maximum temperature burning, important indicators of flame. The completeness of combustion of combustible components will depend on the quality of this component, and the oxidative, reducing characteristics obtained by the flame will depend on its quantity.
The storage conditions for gases are subject to special requirements. The use of special containers (cylinders) is mandatory, as:
If atmospheric oxygen is used, the welds will not work out evenly. In this case, after melting and subsequent connection, the metal will lose its original quality. The use of standard oxygen, which is contained in the atmosphere is not effective enough. It contains a variety of impurities that significantly reduce the rate of combustion of the components, and this accordingly affects the temperature of the flame of the burner.

Important! The proportions of gas mixtures must be observed when using any type of gas. The choice itself will depend on the material being welded. For example, for joining steel samples, the gas composition should contain 18% carbon dioxide, and for joining stainless steel materials, the mixture should be 98% argon.
Mechanized welding in a shielding gas environment involves the use of active, inert gases. They do not dissolve in metals, are not poisonous.
Varieties of gases:
Active gases protect against air in the weld area. They react, dissolve in metals.
The most popular gas mixtures, which increase the quality of the seam, improve the joining process:
Shielded gas welding is characterized by melting of the material. The process itself is based on the connection of the individual elements of a preheated metal prior to melting. For this, a high-temperature flame of the burner is taken, which is formed during the combustion of the gas composition with oxygen. The gap between the samples is filled with pre-molten metal wire.
Benefits:
Disadvantages:
Despite some drawbacks, welding in shielding gases allows an experienced welder, with the correct burner flame power and concentration of the gas mixture, to make welded joints of high quality.
With a relatively slow heating of a metal sample, an insignificant concentration of heat during heating, gas welding performance significantly decreases with increasing thickness of the metal products that are connected.
Example: if the thickness of the welded steel sheet 0.1 cm, the gas welding speed is approximately 10.0 m / h, if the material thickness is 1 cm, the speed is not more than 2.0 m / h.
Welding in shielding gases of steel products, the thickness of which exceeds 0.6 cm, is less effective when compared with arc welding. In such cases, it is rarely used.
The price of gas along with oxygen is greater when compared with the price of the electricity used when using contact, arc welding. 
Gas welding is more difficult for automatic and mechanical processes than electric welding. Therefore, automated gas welding with multi-flame burners is used only when connecting thin metal pipes, shells.

Welding in a protective gas environment provides the ability to weld almost any metal that is used on technical equipment. For example, lead, copper, cast iron are more amenable to gas welding than electric arc. And due to the simplicity of design, gas-welding equipment is quite in demand in agriculture, at engineering enterprises, when performing repair and construction works, and other areas of activity.
When choosing gas for welding for an individual situation, it is recommended to consider the following criteria:
Gas welding will cost an order of magnitude higher than arc, contact electric welding, since gas with oxygen is much more expensive than electricity.
This method of joining metal parts, such as gas welding, has been around for more than a hundred years. During this time, this technology continues to improve successfully, although other welding methods that use an electric arc develop more actively and displace welding in which a gas torch is used.
This method of joining metals, such as gas welding, involves the melting of the materials to be joined, as a result of which a homogeneous structure is formed. Gas combustion, due to which heating and molten metal is carried out, is ensured by introducing pure oxygen into the gas mixture. This method of joining metals has a number of advantages.
This method also has disadvantages.

Gas welding technology involves the use of various types of gases, the choice of which depends on a number of factors.
One of the gases used for welding is oxygen. This gas is characterized by the absence of color and odor; it acts as a catalyst, activating the melting processes of the material being joined or cut.
In order to store and transport oxygen, special cylinders are used in which it is kept under constant pressure. Upon contact with technical oil, oxygen can ignite, so the very possibility of such contact should be excluded. Cylinders containing oxygen must be stored in rooms protected from heat and sunlight.
Welding oxygen is obtained by isolating it from ordinary air, for which purpose special devices are used. Depending on the degree of its purity, oxygen is of three types: the highest (99.5%), the first (99.2%) and the second (98.5%) grade.
For various manipulations with metals (welding and cutting), the colorless acetylene gas C2H2 is also used. Under certain conditions (pressures in excess of 1.5 kg / cm2 and temperatures above 400 degrees), this gas can explode spontaneously. Acetylene is obtained by the interaction of calcium carbide and water.
The advantage of using acetylene in metal welding is that its combustion temperature allows this process to be carried out without problems. Meanwhile, the use of cheaper gases (hydrogen, methane, propane, kerosene vapors) does not make it possible to obtain such a high combustion temperature.
To carry out welding of metals, in addition to gas, are also necessary. It is due to these materials that a weld is created, all its characteristics are formed. The wire used for welding must be clean, without signs of corrosion and paint on its surface. In some cases, a strip of the same metal that is being welded can be used as such a wire. In order to protect the weld pool from external factors, it is necessary to use a special flux. Boric acid and borax are often used as such a flux, which are applied directly to the surface of the metal being welded or to the wire used for welding. Without flux, gas can be performed, and when connecting parts from aluminum, copper, magnesium and their alloys, such protection is necessary.
Gas welding technology involves the use of certain equipment.

Water shutter
A water shutter is necessary to ensure the protection of all elements of the equipment (acetylene generator, pipes) from the reverse draft of the fire from the burner. Such a shutter, in which water should be at a certain level, is placed between the gas burner and the acetylene generator.
Gas cylinderSuch cylinders are painted with different colors, depending on what gas they plan to store. Meanwhile, the upper part of the container is not painted to prevent gas from contacting the components of the paint. It should also be borne in mind that valves made of copper cannot be installed on cylinders that store acetylene, as this can lead to a gas explosion.
GearboxIt is used to reduce the pressure of the gas leaving the cylinder. Reducers can be direct or reverse action, and for liquefied gas models with fins are used, which exclude its freezing when leaving.
Special hosesGas welding cannot be performed without the use of special hoses, through which both gas and flammable liquids can be supplied. Such hoses are divided into three categories, marked by 1) a red strip (operating at a pressure of up to 6 atmospheres), 2) a yellow strip (for supplying flammable liquids), 3) a blue strip (operating at a pressure of up to 20 atm).
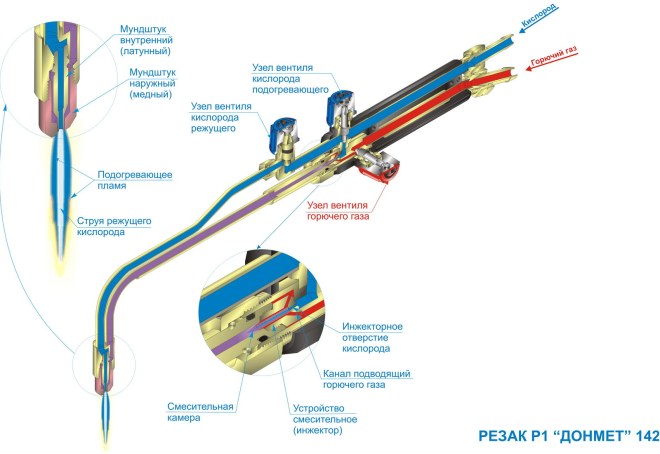
The mixing of gases and their combustion is ensured by the use of a burner, which can be of the injection and non-injector types. Burners are classified by their power, which characterizes the amount of gas passed per unit time. So, there are burners of large, medium, small and small power.
Special tableGas welding is carried out in a specially equipped place called a post. In fact, such a place is a table, which can be with a rotary or fixed tabletop. This table, equipped with exhaust ventilation and everything necessary for storing auxiliary tools, greatly facilitates the work of the welder.
Adjustment of the flame parameters is carried out using a gearbox, which allows you to change the composition of the gas mixture. Using a gearbox, you can get a flame of three main types: reducing (used for welding almost all metals), oxidizing and with an increased amount of combustible gas. When welding metals in a molten bath, two processes occur simultaneously - oxidation and reduction. At the same time, when welding aluminum and magnesium, oxidative processes proceed more actively.
The weld itself and the area adjacent to it are characterized by different parameters. So, the metal section adjacent to the seam is characterized by minimal strength, it is he who is most prone to destruction. The metal adjacent to this zone has a structure with large grains.

To improve the quality of the seam and the area adjacent to it, perform additional heating or the so-called thermal forging of the metal.
Welding technologies for various metals have their own nuances.
Do not enter into chemical interaction with metals and practically do not dissolve in metals
Argon (Ar) - colorless, odorless, non-combustible, non-toxic gas, almost 1.5 times heavier than air. In metals, it is insoluble in both liquid and solid states. (-79) Two grades are produced: the highest and the first.
The highest grade gas contains 99.993% argon, not more than 0.006% nitrogen and not more than 0.0007% oxygen. It is recommended for welding critical metal structures from active and rare metals and alloys, non-ferrous metals.
The first grade gas contains 99.98% argon, up to 0.01% nitrogen and not more than 0.002% oxygen. Recommended for welding steel and pure aluminum.
Helium (Not) - colorless gas, odorless, non-toxic, much lighter than air and argon. Two grades are produced (-75): high purity (up to 99.985%) and technical (99.8%).
It is used less often than argon, due to its scarcity and high cost. However, at the same current value, the arc in helium releases 1.5–2 times more energy than in argon. This contributes to a deeper penetration of the metal and a significant increase in welding speed.
Helium is used in welding chemically pure and active materials, as well as alloys based on aluminum and magnesium.
Nitrogen (N 2) - gas without color, odor and taste, non-toxic. It is used only for welding copper and its alloys, in relation to which nitrogen is an inert gas. Four grades are produced (-74): the highest - 99.9% nitrogen; 1st - 99.5%; 2nd - 99.0%; 3rd - 97.0%.
They protect the welding zone from air, but they themselves dissolve in liquid metal or enter into chemical interaction with it
Oxygen (O 2) - gas without color, smell and taste. Non-combustible, but actively supports combustion. Technical gaseous oxygen (GOST5583-78) is available in three grades: 1st grade - 99.7% oxygen; 2nd - 99.5%; 3rd - 99.2%. It is used only as an additive to inert and active gases.
Carbon dioxide (CO 2) - colorless, with a faint odor, with pronounced oxidizing properties, is well soluble in water. Heavier than air by 1.5 times, can accumulate in poorly ventilated areas, in wells, pits. Three grades are produced (-85): the highest is 99.8% СО 2, the 1st is 99.5% and the 2nd is 98.8%. Grade 2 carbon dioxide is not recommended. To reduce the humidity of CO2, it is recommended to install the cylinder with the valve down and after 1-2 hours open the valve for 8-10 seconds to remove water. Before welding, a small amount of gas is discharged from a normally installed cylinder to remove air trapped inside.
Cast iron, low- and medium-carbon, low-alloy structural corrosion-resistant steels are welded in carbon dioxide.
Serve to improve the welding process and the quality of the weld
A mixture of argon and helium. Optimum composition: 50% + 50% or 40% argon and 60% helium. Suitable for welding aluminum and titanium alloys.
A mixture of argon and oxygen when the oxygen content of 1-5% stabilizes the welding process, increases the fluidity of the weld pool, the transfer of electrode metal becomes droplet. The mixture is recommended for welding carbon and stainless steels.
A mixture of argon and carbon dioxide. The rational ratio is 75-80% of argon and 20-25% of carbon dioxide. At the same time, minimal spraying, high-quality formation of the seam, increase in productivity, good properties of the welded joint are ensured. It is used for welding low-carbon and low-alloy structural steels.
A mixture of carbon dioxide and oxygen. Optimum composition: 60-80% carbon dioxide and 20-40% oxygen. Increases the oxidizing properties of the protective environment and the temperature of the liquid metal. When this mixture is used electrode wire with a high content of deoxidizers, for example Sv-08G2STs. The seam is formed slightly better than when welding in pure carbon dioxide. The mixture is used for welding carbon, alloy and some high alloy structural steels.
A mixture of argon, carbon dioxide and oxygen - a three-component mixture provides high process stability and avoids porosity of the seams. Optimum composition: 75% argon, 20% carbon dioxide and 5% oxygen. It is used in welding carbon, stainless and high alloy structural steels.